Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Co-Infection of Hepatitis B and Hepatitis C in a Patient with Recent Vaccination
*Corresponding author: Leonard Ranasinghe MD, PhD, Professor of Emergency Medicine and Director of Fourth-Year Electives and Sub-Internships, California Northstate University College of Medicine, Elk Grove, CA, USA.
Received: June 12, 2024; Published: June 19, 2024
DOI: 10.34297/AJBSR.2024.22.003029
Abstract
Hepatitis B (HBV) and Hepatitis C (HCV) are viruses of significant global health concern as they can cause serious liver injury such as cirrhosis and hepatocellular carcinoma, while also contributing to extrahepatic symptoms such as pulmonary embolism and portal vein thrombosis. Both viruses are transmitted through similar mechanisms including but not limited to: intravenous (IV) drug use, sexual transmission, perinatal transmission, and transfusion of contaminated blood products. These viruses are endemic in various third world countries, notably Pakistan, where a significant portion of the population is infected by one or both viruses each year. There is currently no manufactured vaccine to prevent the spread of HCV; however, a subunit vaccine exists to prevent the spread of HBV by creating antibodies against the HBsAg antigen. This has been shown to be fully effective in nearly all individuals who receive all doses. Here, we present a case in which a 75 year old woman received a HBV vaccine prior to traveling to Pakistan; however, upon returning to the United States, she presented with symptoms that were suggestive of a HBV and HCV co-infection. We find that the age of the patient, the timing of vaccine administration prior to travel, and a compromised immune system can contribute to the development of HBV, despite vaccination status. Therefore, these are all risk factors that clinicians should be aware of when administering the HBV vaccine and counseling patients about its effects, especially to those traveling to regions endemic for HBV and HCV.
Keywords: Hepatitis, Vaccination, Co-infection of Hepatitis B & C, Immunity, Endemic hepatitis
Introduction
Hepatitis B (HBV), a double stranded Deoxyribonucleic Acid (DNA) virus, and Hepatitis C (HCV), an enveloped Ribonucleic Acid (RNA) virus, are a significant global health concern, and are viruses that belong to the Hepadnaviridae and Flaviviridae classes respectively [1]. They are primarily transmitted through exposure to infected bodily fluids, which may typically occur via sexual transmission, pregnancy, birthing, or sharing of intravenous (IV) drug-injection equipment [2]. Often, HBV and HCV infections can lead to chronic liver disease and hepatocellular carcinoma. Risk factors for HBV contraction include incarcerated individuals, HBV Surface Antigen (HBsAg) positive household contacts, men who have sex with men, IV drug users, healthcare workers, persons with HCV, travelers to countries endemic for HBV, and persons with chronic liver disease. Some risk factors for the contraction of HCV include use of recycled syringes, IV drug use, transfusion of contaminated blood products, sexual transmission, ear and nose piercings, and perinatal transmission [3]. Risk of co-infection of both HBV and HCV is higher in endemic areas such as third world countries like Pakistan [4]. Due to the elusive mechanism of action of HCV, no vaccine has been manufactured to reduce its spread [5]. Contemporary HBV utilizes a subunit mechanism targeting the outer protein coat. Intramuscular injection of this vaccine, usually to the deltoid muscle, confers active immunity against the virus. Newly approved vaccination regimens for adults in the United States require two doses within a one month period [3]. Once an individual has received the HBV vaccine, the subunit vaccine induces antibody production against the HBsAg antigen, thereby conferring immunity against HBV. However, in a few rare instances, individuals may still contract HBV despite having already been vaccinated against the virus [6,7].
The primary objective of this study is to present a case of an elderly woman who was vaccinated against HBV prior to traveling to Pakistan; however, upon returning to the United States, she presented to the Emergency Department (ED) with symptoms suggestive of an infection of both HBV and HCV.
Case Presentation
A 75-year-old female with a medical history of HBV and HCV was admitted to the ED for abdominal pain. She had previously received a vaccination for HBV, and prior to the visitation she had recently traveled to Pakistan. Upon admittance to the ED, the patient presented with mild edema in both lower extremities and a cough that had been persistent for four days. She also reported some recent episodes of epistaxis. The patient was afebrile with a temperature of 98.5°C, stable heart rate of 85 beats per minute, breathing rate of 17 breaths per minute, and 99% oxygen saturation on room air. Her vitals were only remarkable for an elevated blood pressure of 170/71mmHg. The physical examination revealed edema in the lower extremities, which had been ongoing for several weeks, but without calf tenderness or altered range of motion. There were no pulsatile masses or tenderness felt in the abdomen, nor was there any spinal or hip-knee tenderness. In addition, the patient was negative for nausea, vomiting, chest pain, headache, difficulty with breathing, and paresthesia. The rest of her physical examination and review of systems showed no abnormalities.
Laboratory investigations notably revealed elevated levels of alanine aminotransferase (387U/L, N=7-56U/L), aspartate aminotransferase (495 U/L, N=8-33U/L), hepatic alkaline phosphatase (304U/L, N=44-147U/L), total bilirubin (7.9mg/dL, N=0.1-1.2mg/dL), and direct bilirubin (2.1mg/dL, N=0.1-1.2mg/dL). There was also a remarkably elevated d-dimer (19.54ug/mL, N<0.25ug/mL), prothrombin time (18.2seconds, N=11-13.5seconds), INR (1.47, N</=1.1), activated partial thromboplastin time (40.7seconds, N=60-70 seconds), and red cell distribution width (48.9fL, N=39-46fL). Several other laboratory tests were within normal limits, including lipase (N=23-300U/L), creatine kinase (N=30-135U/L), and creatinine (N=0.52-1.04mg/dL). All laboratory results are summarized in Table 1. Based on her electrocardiogram, she had a corrected QT interval of 454ms, which is within normal limits. Urinalysis showed trace amounts of leukocytes, ketones, and proteins, along with moderate detection of bilirubin (Table 1).
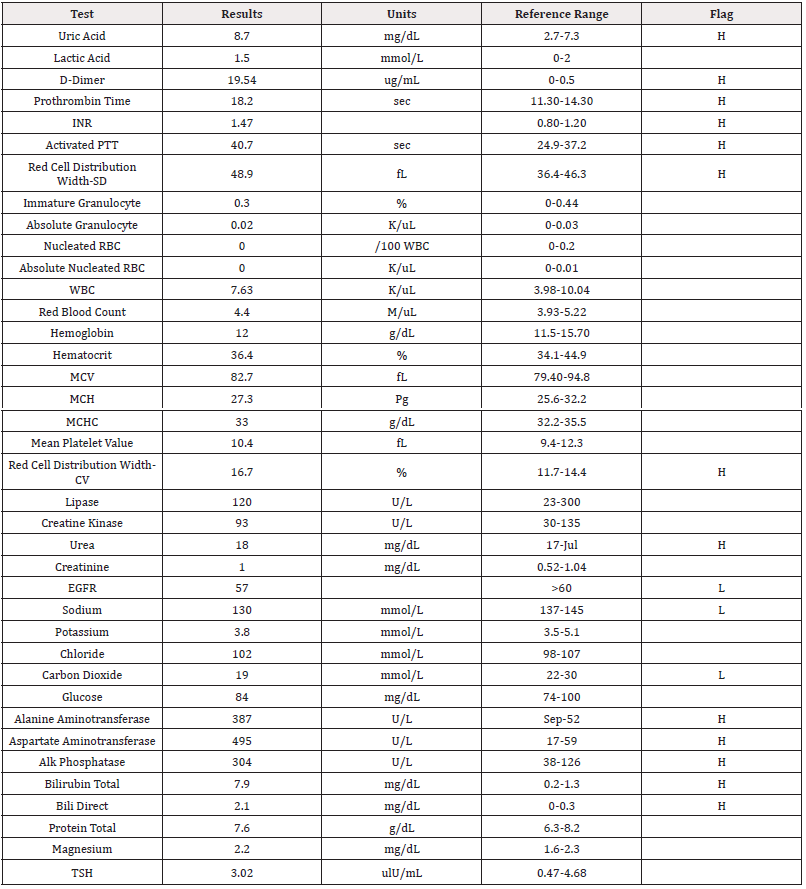
Table 1: Laboratory Values.
Note*: INR: international normalized ratio, PTT: partial thromboplastin time, RBC: red blood cell, WBC: white blood cell, MCV: mean corpuscular volume, MCH: mean corpuscular hemoglobin, MCHC: mean corpuscular hemoglobin concentration, EGFR: estimated glomerular filtration rate, TSH: thyroid stimulating hormone.
A Computed Tomographic (CT) image was taken of the patient’s chest using contrast. The scan notably displayed cardiomegaly with diffuse ground-glass opacity, alongside a right main pulmonary artery thrombus that has extended into lower lobe subsegmental pulmonary arteries. There were no other remarkable findings in this image, including any indications of pericardial effusion, lymphadenopathy, or abnormalities in the aorta. The CT image of the abdomen and pelvis exhibited hepatitis cirrhosis and numerous intrahepatic masses, which correlate with extensive hepatic metastasis. There is a portal vein thrombus that has extended into the main, left, and right portal veins, as well as the superior mesenteric vein. The image also showed recanalization of the umbilical vein. Aside from a 1.2cm left upper pole renal cyst, there were no other abnormalities, including no adrenal or pancreatic masses, splenomegaly, or signs of appendicitis.
Given that the patient did not have any recent extensive bleeding, she was given intravenous (IV) administration of heparin, along with IV fluids. She was kept in the ED for observation, and was eventually admitted to telemetry for monitoring of hepatic metastatic disease. The patient was provided with instructions to follow-up with her primary-care physician to review symptoms and undergo further urine screening based on the trace protein detected. She was also referred to a gastroenterology surgery clinic to review her hepatic findings, and a hematology-oncology clinic to address the abnormal hematological laboratory results.
Discussion
Chronic HCV is a viral liver infection that affects 71 million individuals across the world and can lead to long-term health problems including liver damage, liver failure, cirrhosis, liver cancer, and death. The spread of HCV occurs most commonly in third-world countries such as Pakistan, and it occurs primarily through IV drug use, street barbers, unsafe blood transfusions, use of unsterilized surgical instruments and recycled syringes [3]. Despite the large number of individuals affected by HCV, there is currently no preventative vaccine that has been manufactured to reduce its spread. One reason for the lack of a HCV vaccine is due to the elusive mechanism of action of the virus. HCV is known to have marked genetic diversity and multiple mechanisms of persistence in its hosts. Furthermore, individuals infected with HCV have been shown to have a poor immune response against the virus [5]. These factors have not only made manufacturing a preventative vaccine against HCV difficult, but they have also led to hosts having a compromised immune system due to HCV infection [8].
Chronic HBV is a viral infection that affects more than two billion individuals across the world [9]. Similar to HCV, HBV can also lead to long-term health problems which include liver damage, cirrhosis, and hepatocellular carcinoma [4]. HBV has been linked to the development of extrahepatic health problems such as portal vein thrombosis and the development of pulmonary emboli [10]. HBV shares similar modes of transmission as HCV including perinatal infection, skin and mucous membrane infections from contaminated blood or body fluids, or via sexual contacts [11]. Because they share common modes of transmission, dual infection with HBV and HCV is possible, especially in endemic areas such as Pakistan [4]. Although the exact mechanism is yet to be determined, it is known that co-infection with both viruses can lead to a severe course of liver disease, while predisposing these individuals to a significantly high risk of hepatocellular carcinoma [4]. Furthermore, despite there being no current HCV vaccine that exists, a recombinant subunit vaccine does exist for HBV. The HBV vaccine introduces recombinant viral HBsAg into the recipient’s body. The recombinant HBsAg particles differ from natural ones since the recombinant HBsAg particles lack the preS domain and glycosylation due to being produced in yeast cells. The introduction of the recombinant HBsAg particles leads to an immunogenic response in the body, eventually allowing for the production of antibodies against the HbsAg antigen. This thereby confers immunity against HBV. Administration of vaccines against HBV remains the most effective way to curb the spread of the deadly virus [12].
Despite the existence of a vaccine that can reduce the spread of HBV, in some rare instances, individuals may still contract the virus despite prior vaccination. This more commonly results in a small subset of patients who may have had a suboptimal immunological response to vaccination, or in individuals who were vaccinated as infants and had their immunity wane over time [6]. Another important factor to consider when discussing the protective effects of the HBV vaccine is that it takes on average one month after the completion of all vaccine doses for antibodies to develop against the HBsAg antigen [7]. Finally, the last aspect to consider is the involvement of aging and consequent worsening of their immune responses [13]. In this particular case, a 75-year old female patient was vaccinated for HBV prior to traveling to Pakistan. Due to the patient’s age, there is a high likelihood that the patient did not generate sufficient immunity against the HBsAg antigen, despite having received a vaccine against HBV. Furthermore, there may not have been enough time between when the vaccine was administered and when the patient contracted HBV for full antibody development against the HBsAg antigen to have occurred. Finally, the fact that the patient traveled to Pakistan, a country that is endemic to both HBV and HCV, put this patient at a high risk of co-infection. As mentioned, while the exact mechanism of action of how HBV and HCV co-infection occurs has not been deduced, it can be hypothesized that the HCV infection may have left the patient immunocompromised, thereby leading to the patient simultaneously contracting HBV despite having been vaccinated against the virus.
Conclusion
In summary, this case highlights the risk factors associated with contracting HBV and HCV upon traveling to Pakistan, a region endemic to both viruses. This case demonstrates how age, the timing of vaccine administration prior to travel, and how a compromised immune system can lead to the development of HBV despite being vaccinated against it. It is critical for healthcare providers to take these risk factors into consideration when administering the HBV vaccine and counseling patients about its protective effects, especially if patients are traveling to third-world countries, such as Pakistan, that are endemic to HBV and HCV.
Acknowledgements
None.
Conflicts of Interest
None.
References
- Saeed U, Waheed Y, Ashraf M (2014) Hepatitis B and hepatitis C viruses: A review of viral genomes, viral induced host immune responses, genotypic distributions and worldwide epidemiology. Asian Pacific Journal of Tropical Disease 4(2): 88-96.
- Yang S, Wang D, Zhang Y, Yu C, Ren J, et al. (2015) Transmission of Hepatitis B and C Virus Infection Through Body Piercing: A Systematic Review and Meta-Analysis. Medicine 94(47): e1893.
- Tariq M, Shoukat AB, Akbar S, Hameed S, Naqvi MZ, et al. (2022) Epidemiology, risk factors, and pathogenesis associated with a superbug: A comprehensive literature review on hepatitis C virus infection. SAGE Open Med 10: 20503121221105957.
- Caccamo G, Saffioti F, Raimondo G (2014) Hepatitis B virus and hepatitis C virus dual infection. World J Gastroenterol 20(40): 14559-14567.
- Liang TJ (2013) Current progress in development of hepatitis C virus vaccines. Nat Med, 19(7): 869-878.
- Su TH, Chen PJ (2012) Emerging hepatitis B virus infection in vaccinated populations: A rising concern? Emerg Microbes Infect 1(9): e27.
- Di Lello FA, Martínez AP, Flichman, DM (2022) Insights into induction of the immune response by the hepatitis B vaccine. World J Gastroenterol 28(31): 4249-4262.
- Urbanowicz A, Zagożdżon R, Ciszek M (2019) Modulation of the Immune System in Chronic Hepatitis C and During Antiviral Interferon-Free Therapy. Arch Immunol Ther Exp 67(2): 79-88.
- MacLachlan JH, Cowie BC (2015) Hepatitis B Virus Epidemiology. Cold Spring Harb Perspect Med 5(5): a021410.
- Galli L, Gerdes VEA, Guasti L, Squizzato A (2014) Thrombosis Associated with Viral Hepatitis. J Clin Transl Hepatol 2(4): 234-239.
- Kwon SY, Lee CH (2011) Epidemiology and prevention of hepatitis B virus infection. Korean J Hepatol 17(2): 87-95.
- Pattyn J, Hendrickx G, Vorsters A, Van Damme P (2021) Hepatitis B Vaccines. J Infect Dis 224(12 Suppl 2): S343–S351.
- Lee JL, Linterman MA (2022) Mechanisms underpinning poor antibody responses to vaccines in ageing. Immunol Lett 241: 1-14.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.